HHS Orders 2.5 Million More Doses of JYNNEOS Vaccine For Monkeypox Preparedness. The U.S. Division of Health and Human Services (HHS) today requested an extra 2.5 million doses of Bavarian Nordic’s JYNNEOS, a FDA-authorized vaccine showed for counteraction of smallpox and monkeypox, for use in answering current or future monkeypox episodes and as a component of U.S. smallpox preparedness. Conveyances from this most recent request will start showing up at the Strategic National Stockpile (SNS) in the not so distant future and will go on through mid 2023.
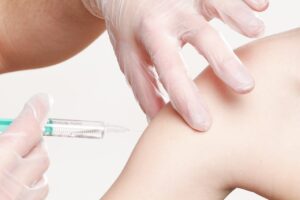
The unexpected inventory of vaccines is essential for the Biden-Harris Administration’s more extensive system to battle the monkeypox infection and safeguard those most in danger, including by speeding up the creation and conveyance of vaccines, making testing more available and helpful, and discussing consistently with wellbeing and local area pioneers about the infection.
“We are working nonstop with general wellbeing officials in states and huge metro regions to give them vaccines and medicines to answer the current monkeypox episode,” said HHS Secretary Xavier Becerra. “This request for extra JYNNEOS vaccine will assist us with pushing out more vaccine rapidly, realizing that we have more doses on the way before very long – and is just conceivable on account of our longstanding interest in smallpox and monkeypox preparedness.”
The request declared today is notwithstanding the 500,000 doses of government-claimed vaccine the organization is creating in 2022 for use in the ongoing reaction to monkeypox in the U.S and brings the absolute vaccine doses to be conveyed in 2022 and 2023 to more than 4 million. The organization will deliver these doses in fluid frozen form utilizing vaccine previously made in mass under a current 10-year contract with the Biomedical Advanced Research and Development Authority (BARDA) Doom Is Now Playable, inside the HHS Office of the Assistant Secretary for Preparedness and Response (ASPR); that agreement was essential for continuous public preparedness efforts against smallpox.
“The clinical countermeasures accessible to help answer the ebb and flow flare-up are the consequence of long stretches of venture and arranging made conceivable through the continuous work among HHS and confidential industry,” said Gary Disbrow, overseer of the Biomedical Advanced Research and Development Authority. “We are satisfied that we have had the option to work with our accomplices at Bavarian Nordic to speed up conveyance of vaccines that can assist with keeping individuals safe and stem the spread of the infection.”
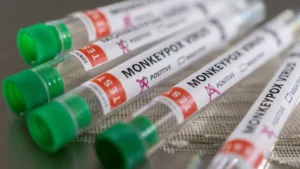
BARDA upheld the advancement of JYNNEOS, which is supported by the FDA to forestall smallpox and monkeypox. The U.S. government claims sufficient smallpox vaccine – JYNNEOS and ACAM2000 – to immunize millions of Americans, if necessary.
As of June 24, ASPR’s SNS held roughly 65,000 doses of JYNNEOS in prompt stock with conveyance of 300,000 extra doses before long. On June 28, HHS reported that it would promptly make accessible 56,000 doses and not long after would make accessible 240,000 extra doses. The SNS additionally has more than 100 million doses of ACAM2000 which was created with SNS support and is endorsed by FDA for use in forestalling smallpox. The Centers for Disease Control and Prevention (CDC) as of now has an extended admittance Investigational New Drug convention which permits utilization of ACAM2000 for monkeypox.
What’s more, the SNS has over 1.7 million treatment courses of the smallpox antiviral medication TPOXX, which was created with BARDA support and can be utilized to treat people with monkeypox under a fitting administrative component. CDC at present has an extended admittance Investigational New Drug convention which permits its utilization for monkeypox.
